Abstract
The JAK1/2 inhibitor ruxolitinib is a cornerstone of management for some subsets of BCR-ABL negative myeloproliferative neoplasms (MPN). While many patients benefit with reduced splenomegaly and symptoms, some patients respond suboptimally to ruxolitinib, and even patients with a good initial response often develop resistance to ruxolitinib after several years of therapy. Here, we evaluated the efficacy of micheliolide (MCL), a natural guaianolide sesquiterpene lactone, in the JAK2V617F positive MPN entities.
We firstly evaluated the effect of MCL treatment on cell growth and survival in human MPN cell lines expressing the JAK2V617F mutation. MCL alone reduced cell viability and induced apoptosis of UKE1 and SET2 cells in a dose-dependent manner, and obviously improved the effect of ruxolitinib as monotherapy. Importantly, treatment with MCL also induced significant apoptosis of UKE1 cells which were selected for resistance against ruxolitinib.
We further explored the therapeutic potential of MCL in vivo using a Jak2V617F knock-in (Jak2VF) mouse model, and found that DMAMCL, an orally available derivative of MCL, significantly increased ruxolotinib efficacy in Jak2VF mice, manifested as amplified hematological and spleen responses. What's more, DMAMCL, whereas not ruxolitinib, contributed to reduce the in vivo Jak2V617F mutant allele burden reflected by the percentage of mutant hematopoietic cells in peripheral blood and bone marrow.
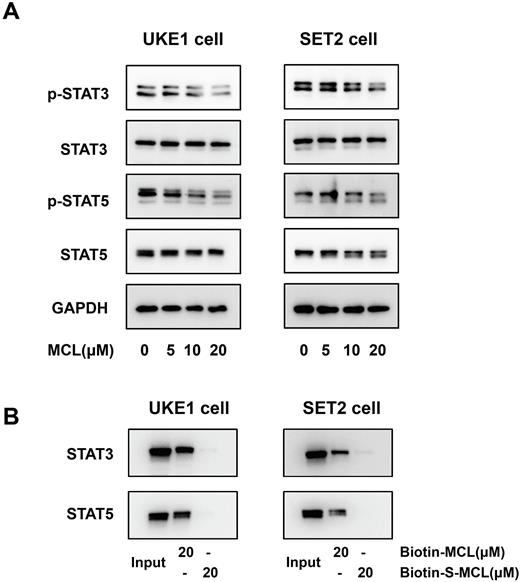
To determine the molecular mechanisms by which MCL exerts efficacy in MPN, we firstly performed western blot (WB) anlyses to evaluate the effect of MCL on hyper-activated JAK-STAT signaling, which is the cornerstone of MPN pathogenesis. We observed a dose-dependent inhibition of STAT3 and STAT5 phosphorylation caused by MCL treatment in UKE1 and SET2 cells (Figure 1A), and as expected, combination MCL with ruxolitinib provided an enhanced inhibitory effect. By next performing the affinity pull-down experiment, we confirmed that MCL suppressed the activation of STAT3 and STAT5 mainly through directly binding to the proteins (Figure 1B).
We further conducted RNA-sequencing analysis in UKE1 cells treated with MCL and/or ruxolitinib to gain insights into mechanisms involved in MCL effect. Gene set enrichment analysis (GSEA) showed that genes related to TNF-α-NF-κB signaling and inflammatory response pathway were significantly downregulated upon treatment with MCL, that were verified by WB analyses and real-time quantitative PCR. Additionally, GSEA also revealed significant upregulation of genes related to reactive oxygen species (ROS) and unfolded protein response (UPR) pathway, respectively. Both flow cytometry and fluorescent microscopy imaging confirmed the ROS accumulation by MCL treatment. We also determined the activation of PERK branch of UPR pathway caused by MCL, as assessed by increased phosphorylation of eIF2α, and upregulation of pro-apoptotic genes, including ATF4, CHOP and GADD34. Finally, by addition of antioxidant agent, the activation of UPR pathway and increase of apoptosis in UKE1 cells induced by MCL were significantly rescued, indicating that MCL exerts cytotoxic effects mainly through triggering the oxidative stress in MPN cells.
In summary, our results demonstrate that MCL is an effective agent with multiple biological activities in MPN, especially inhibition of STAT3/5 activity via directly binding to the proteins, and may be a promising drug combined with ruxolitinib to benefit patients who have suboptimal response or acquire resistance to ruxolitinib.
Figure 1. MCL inhibits STAT3 and STAT5 phosphorylation through binding to the proteins directly. (A) Western blot analyses are shown for phosphorylated-STAT3 (p-STAT3), STAT3, phosphorylated-STAT5 (p-STAT5) and STAT5 levels in total cell extracts from UKE1 and SET2 cells treated with MCL (5, 10, 20μM) for 3 hours. (B) The biotin-conjugated MCL (biotin-MCL) and its inactive analogue S-MCL (biotin-S-MCL) were prepared as the positive- and negative- probe respectively. UKE1 and SET2 cell lysates were incubated with 20μM biotin-MCL or biotin-S-MCL for 1 hour at room temperature, then the mixtures were separately pulled down with streptavidin-coated agarose beads, and STAT3/STAT5 were detected with WB analysis.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal